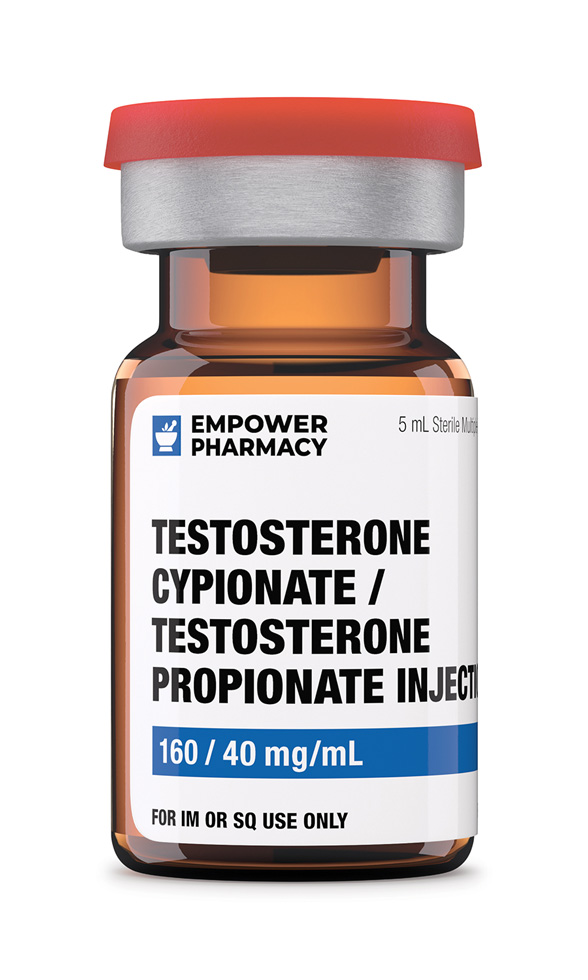
Testosterone Cypionate / Testosterone Propionate Injection
- Description
- Reviews (0)
- Store Policies
- Inquiries
Description
Dosage Strength of Testosterone Cypionate / Testosterone Propionate Injection
Testosterone Cypionate / Testosterone Propionate 160/40 mg/mL 5 mL Vial (Grapeseed Oil)
The first anabolic steroid that was effectively produced was testosterone. A fast-acting, short-ester, oil-based testosterone injectable molecule called testosterone propionate is frequently administered to treat male hypogonadism, or low testosterone levels, and its numerous symptoms.
In order to prolong the therapeutic benefits of synthetic testosterone by delaying its absorption into the bloodstream, testosterone propionate was initially proposed in 1935. Two years later, Schering AG in Germany made it available for therapeutic usage. It was sold under the trade name Testoviron and combined with testosterone enanthate. Prior to 1960, this type of testosterone dominated the market for prescription medications in the United States and was also the first one that was commercially available there.
The main androgen present in the human body is testosterone. Testicular, ovarian, and adrenal cortex cells all produce endogenous testosterone. The management of congenital or acquired hypogonadism involves the use of testosterone therapeutically. For postmenopausal women with breast cancer, testosterone is the most effective exogenous androgen for palliative care. 1938 saw the FDA approve testosterone for use, and 1939 saw its first use. Because of their past illegal use, anabolic steroids—which are testosterone derivatives—are now considered controlled substances. In 1991, testosterone, along with a number of anabolic steroids, received a restricted drug designation. Regular and delayed-release (depot) dose versions of testosterone are both injected intravenously. In September 1995, the FDA initially approved testosterone transdermal patches (Androderm); many transdermal forms and brands are now available including implants, gels, and topical solutions. A testosterone buccal system, Striant, was FDA approved in July 2003; the system is a mucoadhesive product that adheres to the buccal mucosa and provides a controlled and sustained release of testosterone. In May 2014, the FDA approved an intranasal gel formulation (Natesto). A transdermal patch (Intrinsa) for hormone replacement in women is under investigation; the daily dosages used in women are much lower than for products used in males. The FDA ruled in late 2004 that it would delay the approval of Intrinsa women’s testosterone patch and has required more data regarding safety, especially in relation to cardiovascular and breast health.
The Propionate Ester: An ester is any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Most esters are derived from carboxylic acids, and injectable testosterone is typically administered along with one or multiple esters. The addition of a carbon chain (ester) attached to the testosterone molecule controls how soluble it will be once it’s inside the bloodstream. The larger the carbon chain, the longer the ester, and the less soluble the medication; a large/long ester will have a longer half-life. The inverse is true of short carbon chains, like the propionate ester, which acts rapidly upon the body and evacuates the body at a similar rate. With a three-carbon chain, the testosterone ester possesses the shortest half life of all testosterone esters at 4 days.
Sexual development occurs throughout life at all stages of development thanks to endogenous testosterone. It is made synthetically from cholesterol. Androgens play a significant part in the development of males from the time they are fetuses to adulthood. They are essential during puberty. Small amounts of testosterone are also secreted by females from their ovaries. Male sexuality cannot be sustained by the adrenal cortex’s androgen release.
Through a negative-feedback mechanism, elevated androgen plasma concentrations inhibit gonadotropin-releasing hormone (which lowers endogenous testosterone), luteinizing hormone, and follicle-stimulating hormone. Additionally, the production of erythropoietin, the balance of calcium, and blood sugar are all impacted by testosterone. Androgens have a high lipid solubility, which allows them to reach target tissue cells quickly. When testosterone enters cells, it is enzymatically converted to 5-alpha-dihydrotestosterone and joins with cystolic receptors to create a loosely bound complex. The steroid-receptor complex causes cellular alterations in the nucleus and the start of transcription, which are the causes of androgen activity.
RNA polymerase is often stimulated by endogenous androgens, which increases protein synthesis. The growth and maturation of the prostate, seminal vesicle, penis, and scrotum as well as other aspects of typical male sexual development are regulated by these proteins. Androgens promote a dramatic increase in muscle growth and development throughout puberty, as well as a redistribution of body fat. The larynx and vocal cords undergo changes as well, which deepen the voice. The development of the beard and the expansion of body hair mark the end of puberty. The androgens also control the fusion of the epiphyses, the cessation of growth, and the preservation of spermatogenesis. Exogenous androgens must be used in the absence of endogenous androgens to support normal male development and growth.
Breast cancer, sleep apnea, diabetes, heart disease, kidney disease, liver disease, lung disease, prostate cancer or enlargement, any unusual or allergic reactions to testosterone or other medications, being pregnant or trying to become pregnant, or breastfeeding are all conditions that need to be disclosed to your doctor. Your doctor will require routine blood tests while you are using testosterone. The majority of athletic organizations prohibit using this substance on athletes.
Because AndroGel and Striant contain soy ingredients, their manufacturers advise against using those drugs if you have a soy, soybean, or soy lecithin allergy. Utilizing any topical testosterone gel or solution formulation should be done away from flames, fire, and cigarette smoke because topical gels and solutions are frequently flammable. The testosterone undecanoate (Aveed) oil for injection contains refined castor oil and benzyl benzoate, an ester of benzyl alcohol and benzoic acid. In those who have a high sensitivity to polyoxyethylated castor oil, benzoic acid, or benzyl alcohol, testosterone undecanoate use is not recommended.
Patients should be advised to remove the patch before receiving magnetic resonance imaging since some testosterone transdermal systems (like Androderm) contain aluminum or other metal components (MRI). Some transdermal systems have metal components in their backings that could overheat during an MRI and burn the skin where the patch is placed.
Intramuscular injections of testosterone are administered. No intravenous injection should be given. When taken intramuscularly, testosterone undecanoate and testosterone enanthate have both been linked to negative respiratory consequences. Make sure the testosterone is administered into the gluteal muscle slowly and deeply.
Since testosterone can encourage the creation of cancerous tissue, male patients with prostate cancer or breast cancer shouldn’t use it. Patients with prostatic hypertrophy should get careful treatment because androgen therapy may enhance the condition’s symptoms and increase the risk of cancer forming. Elderly people and other patients with clinical or demographic characteristics associated with an increased risk of prostate cancer should be evaluated for the presence of the disease prior to starting testosterone replacement therapy. Following current recommendations for eugonadal men should be the goal of prostate cancer surveillance in patients receiving testosterone therapy. The use of testosterone replacement treatment in geriatric individuals who just have age-related hypogonadism or andropause is not adequately supported by the available research. Furthermore, due to a dearth of elderly participants in controlled trials, the effectiveness and long-term safety of testosterone topical solution in patients older than 65 have not been established. The Beers Criteria state that testosterone is a potentially inappropriate drug (PIM) for geriatric people and that it should be avoided since it may cause cardiac issues and is contraindicated in cases of prostate cancer. Use for moderate to severe hypogonadism is acceptable in the opinion of the Beers expert panel.
Due to impaired drug clearance and an increased risk of drug accumulation, testosterone administration to patients with liver illness or dysfunction should be done with caution. While taking androgens for treatment, edema caused by salt and water retention may also appear. Use testosterone with caution in those who have hepatic disease, renal disease including nephritis and nephrosis, preexisting edema, or cardiac disease such heart failure, coronary artery disease, and myocardial infarction (MI), as fluid retention may make these conditions worse. Research is also being done to determine whether there is a connection between testosterone use and a higher risk of major cardiovascular events, regardless of the presence of pre-existing heart disease. An observational study in the U.S. Veterans Affairs medical system comprised adult male patients with an average age of 60. Patients (n = 8779) undergoing coronary angiography and having low blood testosterone levels of less than 300 ng/dl were included in the retrospective analysis. After a median of 531 days had passed following coronary angiography, 1223 men in the larger group underwent testosterone therapy while 7486 men did not. Patients receiving testosterone therapy had a major and/or fatal cardiovascular event at a rate of 25,7% in the three years following coronary angiography, compared to patients not receiving medication, who had a rate of 19,9%. (Death, stroke, and MI). A second observational study (n = 55,593) looked at the prevalence of acute non-fatal MI after a first testosterone prescription in adult males who were both younger (=55 years) and older (>=65 years). The incidence rate of MI occurring 90 days following the first testosterone prescription was examined and compared to the incidence rate of MI occurring in the year prior to the first prescription. Older males had a 2-fold increased risk of MI within the 90-day window, while younger men with a history of heart disease had a 2- to 3-fold increased risk. In contrast, younger males without a history of heart disease did not show an enhanced risk.The FDA announced at the beginning of 2014 that it would investigate any potential links between testosterone therapy and major cardiovascular events in reaction to these findings. The risk of stroke, MI, or death is not increased by the testosterone therapy that the FDA has approved. However, it is advised that medical professionals carefully consider if the possible risks outweigh the anticipated therapeutic advantages. The FDA will provide its final recommendations and results after the review is complete.
The use of testosterone esters in the treatment of hypogonadal males may worsen sleep apnea, particularly in patients who already have apnea risk factors such obesity or chronic pulmonary illness. There is also limited information available regarding the effectiveness and security of testosterone topical solution and intranasal gel in obese males with BMIs greater than 35 kg/m2.
Patients using high amounts of testosterone run the risk of developing polycythemia. In order to screen for polycythemia, patients receiving testosterone should routinely have their hemoglobin and hematocrit levels tested.
During pregnancy, testosterone is not permitted due to potential harm to the fetus (FDA pregnancy risk category X). Use dependable contraception if you’re a sexually active woman taking testosterone pills. There shouldn’t be any specific need to give the products to women who are in labor or having an obstetric delivery because testosterone is not used throughout pregnancy; safety and efficacy in these situations have not been demonstrated.
Testim testosterone gel is specifically not recommended for use in females; it is solely intended for use in males; and its dosage form gives more testosterone than is recommended for use in females in a number of endocrine problems. Additionally, due to a lack of controlled studies and/or the possibility for virilizing effects, products under the Androgel, Androderm, Aveed, Fortesta, and Striant brands are not recommended for use in females. Patients who are female and taking different types of testosterone therapy need to be properly watched for symptoms of virilization (deepening of the voice, hirsutism, acne, clitoromegaly, and menstrual irregularities). The concurrent use of estrogens does not prevent virilization, which occurs frequently at high levels. While some virilization may be deemed to be acceptable during treatment for breast cancer, medication therapy must be stopped if moderate virilism is visible in order to prevent long-term virilization. Females should be made aware that they can unknowingly come into touch with some testosterone dose forms if they come into contact with a patient who is receiving treatment (i.e., ointments, solutions, and gels). Within 2–12 hours after male test subjects applied gel, vigorous 15-minute intervals of skin-to-skin contact with a female partner resulted in serum female testosterone levels that were > 2 times the female baseline values. When clothing covered the treatment site on the male, testosterone transmission to the female was stopped. Topical testosterone gel was accidentally applied to children who mistakenly touched the treatment area while receiving treatment. A few of the unfavorable side effects stated are pubic hair growth, advanced bone aging, larger genitalia, and aggressive behavior. Most of the patients’ symptoms vanished when exposure to the drug was stopped. However, in a few cases, the advanced bone age and enlarged genitalia did not completely return to normal values. The FDA recommends using safety precautions to lessen the likelihood of unintentional exposure to topical testosterone products. These include washing hands with soap and warm water after each application, covering the application site with clothing, and removing the medication with the same care when it is anticipated that contact with another person will occur. If a non-treated person comes into contact with the region where testosterone was applied, they should wash the area with soap and water as quickly as possible. Most of the patients’ symptoms vanished when exposure to the drug was stopped. However, in a few cases, the advanced bone age and enlarged genitalia did not completely return to normal values. The FDA recommends using safety precautions to lessen the likelihood of unintentional exposure to topical testosterone products. These include washing hands with soap and warm water after each application, covering the application site with clothing, and removing the medication with the same care when it is anticipated that contact with another person will occur. If a non-treated person comes into contact with the region where testosterone was applied, they should wash the area with soap and water as quickly as possible.
It is not recommended for nursing moms to utilize testosterone topical solutions, transdermal patches, or gels. It is advised to steer clear of other testosterone preparations during nursing as well. Uncertainty exists regarding the distribution of testosterone in breast milk and whether exposure would elevate levels above those typically present in human milk. Significant breastfeeding exposure to this androgen may have harmful androgenic effects on the infant, and the medication may also prevent the mother from starting her milk supply properly. In the past, testosterone and androgens were used in conjunction to inhibit lactation. Alternative methods to breastfeeding are recommended in lactating women receiving testosterone therapy.
Androgen therapy, such as testosterone, should be used cautiously in people with diabetes mellitus since it can result in a loss of diabetic control. It is important to monitor blood glucose levels carefully.
Testosterone has been reported to cause osteolysis in people with hypercalcemia, which can be exacerbated in those with metastatic breast cancer, and should be used with caution.
Anaphylactoid reactions as well as significant pulmonary oil microembolism (POME) reactions have been linked to the administration of testosterone undecanoate. During or right away following a 1000 mg intramuscular injection of testosterone undecanoate, incidences of POME responses have been reported. Coughing, the desire to cough, dyspnea, hyperhidrosis, throat constriction, chest pain, disorientation, and syncope were among the symptoms. However, some cases prolonged up to several hours and required emergency care and/or hospitalization. The majority of cases lasted a few minutes and were treated with supportive measures. Clinicians should take care to inject testosterone undecanoate deeply into the gluteal muscle and prevent intravascular injection. In addition to POME reactions, instances of anaphylaxis, including potentially fatal events, have also been documented after testosterone undecanoate injection intramuscularly. It is not advisable to provide testosterone undecanoate again to patients who may have experienced hypersensitive responses. Monitor the patient for 30 minutes following each administration, and in the event of severe POME or anaphylactoid reactions, administer the necessary medical care.
Intranasal formulations of testosterone (e.g., Natesto) are not recommended for individuals with a history of nasal disorders such as nasal polyps; nasal septal perforation; nasal surgery; nasal trauma resulting in nasal fracture within the previous 6 months or nasal fracture that caused a deviated anterior nasal septum; sinus surgery or sinus disease. In addition, the safety and efficacy of intranasal testosterone has not been evaluated in individuals with mucosal inflammatory disorders such as Sjogren’s syndrome. Patients with rhinorrhea (rhinitis) who are receiving intranasal formulations of testosterone may experience decreased medication absorption secondary to nasal discharge. These patients may experience a blunted or impeded response to the intranasal medication. In clinical evaluation, serum total testosterone concentrations were decreased by 21—24% in males with symptomatic allergic rhinitis, whether treated with nasal decongestants or left untreated. Treatment with intranasal testosterone should be delayed until symptoms resolve in patients with nasal congestion, allergic rhinitis, or upper respiratory infection. If severe rhinitis symptoms persist, an alternative testosterone replacement therapy is advised.
Androgel, Axiron, Fortesta, Testim, Striant buccal tablets, Natesto intranasal gel, and Aveed injectable testosterone undecenoate have not been proven to be safe and effective in neonates, babies, children, or teenagers under the age of 18. Depo-Testosterone injection’s safety and effectiveness in children under the age of 12 have not been established, and Androdem patches have not been studied in young patients under the age of 15 years. In general, using testosterone in youngsters should only be done with the utmost caution. Testosterone can hasten bone maturation without promoting linear growth to make up for it, which can sometimes limit adult stature. In order to monitor the rate of bone maturation and the impact of the medication on epiphyseal centers in prepubertal males receiving testosterone, radiographic exams of the hand and wrist should be carried out every six months. Growth stops once the epiphyses have closed. Epiphyseal closure can be increased for several months even after the end of the treatment. Pediatric patients have also unintentionally come into contact with topical testosterone gel after being treated persons had applied it directly to their skin. The negative side effects mentioned include enlarged genitalia, pubic hair growth, advanced bone aging, increased libido, and aggressive conduct. When exposure to the substance was terminated, the majority of patients’ symptoms disappeared. The enlarged genitalia and advanced bone age did not fully revert to expected values in a small number of patients, nevertheless. The FDA advises taking safety measures to reduce the possibility of inadvertent exposure, including covering the application location with clothing, removing the drug with soap and water when coming into touch with another person, and washing hands with soap and warm water after each application. The non-treated person should wash the area with soap and water as soon as possible if they come into direct contact with the location of testosterone application.
During pregnancy, testosterone is not advised due to the potential harm it could do to the growing fetus (FDA pregnancy risk category X). Women who are capable of producing children should utilize trusted contraception when receiving testosterone medication. Because testosterone is not used during pregnancy, there should be no particular reason to provide the items to women who are in labor or having an obstetric delivery; safety and effectiveness in these situations have not been shown.
It is not recommended for nursing moms to utilize testosterone topical solutions, transdermal patches, or gels. It is advised to stay away from other testosterone formulations during nursing as well. Uncertainty exists regarding the distribution of testosterone in breast milk and whether exposure would elevate levels above those typically present in human milk. Significant nursing exposure to this androgen may have harmful androgenic effects on the newborn, and the medication may also prevent the mother from starting her milk supply properly. In the past, testosterone and androgens were used in conjunction to inhibit lactation. Alternative methods to breastfeeding are recommended in lactating women receiving testosterone therapy.
Some diabetes medications and some drugs to treat or prevent blood clots including warfarin, oxyphenbutazone, propranolol, and steroid drugs like prednisone or cortisone are examples of possible interactions. It’s probable that not all combinations are covered by this list.
NOTE: Testosterone is a substrate for hepatic cytochrome P450 (CYP) 3A4 isoenzyme. Testosterone is also both transported by and an inhibitor of P-glycoprotein transport.
Testosterone can increase the anticoagulant action of warfarin. Serious bleeding has been reported in some patients with this drug-drug interaction. Although the mechanism is unclear, testosterone may reduce procoagulant factors. Reduction of warfarin dosage may be necessary if testosterone therapy is coadministered. More frequent monitoring of INR and prothrombin time in patients taking such oral anticoagulants is recommneded, especially at the initiation and termination of androgen therapy. It is unclear if testosterone can augment the anticoagulant response to heparin therapy or if testosterone alters the effect of other non-coumarin oral anticoagulants in a similar manner.
Based on case reports with methyltestosterone and danazol, androgens may increase plasma concentrations of cyclosporine, leading to a greater risk of nephrotoxicity.
Coadministration of corticosteroids and testoterone may increase the risk of edema, especially in patients with underlying cardiac or hepatic disease. Corticosteroids with greater mineralocorticoid activity, such as fludrocortisone, may be more likely to cause edema. Administer these drugs in combination with caution.
Goserelin and leuprolide inhibit steroidogenesis. Concomitant use of androgens with goserelin or leuprolide is relatively contraindicated and would defeat the purpose of goserelin or leuprolide therapy.
Androgens should be provided concurrently with other hepatotoxic drugs with caution because they can enhance the risk of hepatotoxicity. Particularly for those with a history of liver illness, patients should be constantly watched for symptoms of liver damage.
Androgens may be necessary to assist in the growth response to human growth hormone, but excessive doses of androgens in prepubescent males can accelerate epiphyseal maturation.
Erythropoiesis is known to be stimulated by androgens. Despite the fact that endogenous erythropoietin production is suppressed in individuals with chronic renal failure, erythropoietin can still be produced in tissues other than the kidney, albeit in modest levels. Epoetin alfa dosage for treating anemia can be decreased by concurrently administering androgens because they improve the patient’s reaction to the medication. This medicine combination should be avoided if at all feasible since negative effects have been linked to a sudden rise in blood viscosity. It is necessary to assess this combo further.
It would be nonsensical for patients taking androgens to utilize these antiandrogenic medications because the 5-alpha reductase inhibitors (such as dutasteride and finasteride) have antiandrogenic effects that are antagonistic to the actions of androgens.
Drug interactions with Saw palmetto, Serenoa repens have not been specifically studied or reported. Saw palmetto extracts appear to have antiandrogenic effects. The antiandrogenic effects of Saw palmetto, Serenoa repens would be expected to antagonize the actions of androgens; it would seem illogical for patients taking androgens to use this herbal supplement.
Limited data suggest that testosterone concentrations increase during fluconazole administration. It appears that fluconazole doses of 200 mg/day or greater are more likely to produce this effect than doses of 25—50 mg/day. The clinical significance of this interaction is unclear at this time. Although data are not available, a similar reaction may occur with voriconazole. Both fluconazole and voriconazole are inhibitors of CYP3A4, the hepatic microsomal isoenzyme responsible for metabolism of testosterone.
Exogenously administered androgens (testosterone derivatives or anabolic steroids) have variable effects on blood glucose control in patients with diabetes mellitus. In general, low testosterone concentrations are associated with insulin resistance. Further, when hypogonadal men (with or without diabetes) are administered exogenous androgens, glycemic control typically improves as indicated by significant reductions in fasting plasma glucose concentrations and HbA1c. In one study in men with diabetes, testosterone undecenoate 120 mg PO/day for 3 months decreased HbA1c concentrations from a baseline of 10.4% to 8.6% (p < 0.05); fasting plasma glucose concentrations decreased from 8 mmol/l at baseline to 6 mmol/l (p < 0.05). Significant reductions in HbA1c and fasting plasma glucose concentrations did not occur in patients taking placebo. Similar results have been demonstrated with intramuscular testosterone 200 mg administered every 2 weeks for 3 months in hypogonadal men with diabetes. In healthy men, testosterone enanthate 300 mg IM/week for 6 weeks or nandrolone 300 mg/week IM for 6 weeks did not adversely affect glycemic control; however, nandrolone improved non-insulin mediated glucose disposal. It should be noted that some studies have shown that testosterone supplementation in hypogonadal men has no effect on glycemic control. Conversely, the administration of large doses of anabolic steroids in power lifters decreased glucose tolerance, possibly through inducing insulin resistance. While data are conflicting, it would be prudent to monitor all patients with type 2 diabetes on antidiabetic agents receiving androgens for changes in glycemic control, regardless of endogenous testosterone concentrations. Hypoglycemia or hyperglycemia can occur; dosage adjustments of the antidiabetic agent may be necessary.
In vitro, both genistein and daidzein inhibit 5 alpha-reductase isoenzyme II, resulting in decreased conversion of testosterone to the potent androgen 5-alpha-dihydrotestosterone (DHT) and a subsequent reduction in testosterone-dependent tissue proliferation. The action is similar to that of finasteride, but is thought to be less potent. Theoretically, because the soy isoflavones appear to inhibit type II 5-alpha-reductase, the soy isoflavones may counteract the activity of the androgens.
Conivaptan is a potent inhibitor of CYP3A4 and may increase plasma concentrations of drugs that are primarily metabolized by CYP3A4. Testosterone is a substrate for CYP3A4 isoenzymes. The clinical significance of this theoretical interaction is not known.
Testosterone is an inhibitor of P-glycoprotein transport. Ranolazine is a substrate of P-glycoprotein, and inhibitors of P-glycoprotein may increase the absorption of ranolazine. In addition, ranolazine inhibits CYP3A and may increase plasma concentrations of drugs that are primarily metabolized by CYP3A4 such as testosterone.
Ambrisentan is a substrate for P-glycoprotein transport, an energy-dependent drug efflux pump. The inhibition of P-glycoprotein, by drugs such as testosterone, may lead to a decrease in the intestinal metabolism and an increase in the oral absorption of ambrisentan. If ambrisentan is coadministered with a P-glycoprotein inhibitor, patients should be monitored closely for adverse effects.
Coadministration of oxyphenbutazone and testosterone may lead to elevated concentrations of oxyphenbutazone. Monitor patients for adverse effects when coadministering these drugs together.
Testosterone cypionate has been shown to increase the clearance of propranolol in one study. Monitor patients taking testosterone and propranolol together for decreased therapeutic efficacy of propranolol.
Coadministration of dabigatran and testosterone may result in increased dabigatran serum concentrations, and, therefore, an increased risk of adverse effects. Coadministration of dabigatran and testosterone should be avoided in patients with severe renal impairment (CrCl 15—30 ml/min). Dabigatran is a substrate of P-gp; testosterone is a P-gp inhibitor. P-gp inhibition and renal impairment are the major independent factors that result in increased exposure to dabigatran.
Concomitant use of testosterone, a P-glycoprotein (P-gp) inhibitor, and afatinib, a P-gp substrate, may increase the exposure of afatinib.17 If the use of both agents is necessary, consider reducing the afatinib dose if the original dose is not tolerated.
Concomitant use of intranasal testosterone (e.g., Natesto) and other intranasally administered drugs in not recommended; the drug interaction potential between these agents is unknown. Eighteen males with seasonal allergic rhinitis were treated with intranasal testosterone and randomized to receive oxymetazoline (30 minutes prior to intranasal testosterone) or no treatment. In general, serum total testosterone concentrations were decreased by 21—24% in males with symptomatic allergic rhinitis, due to the underlying condition. A mean decrease in AUC and Cmax (2.6% and 3.6%, respectively) for total testosterone was observed in males with symptomatic seasonal rhinitis when treated with oxymetazoline compared to untreated patients. Concomitant use of oxymetazoline does not impact the absorption of testosterone.
This list may not include all possible interactions. Give your health care provider a list of all the medicines, herbs, non-prescription drugs, or dietary supplements you use. Also tell them if you smoke, drink alcohol, or use illegal drugs. Some items may interact with your medicine.
During extended testosterone therapy, male patients may undergo feminization, which is thought to be caused by the suppression of gonadotropin secretion and the conversion of androgens to estrogens. Mastalgia and gynecomastia are two of these symptoms, which are particularly pronounced in male patients with concurrent hepatic illness. Gynecomastia (Testim: 1 percent; Androgel: 1–3 percent) and mastalgia (Androgel: 1–3 percent) were reported during a clinical evaluation of testosterone gel. Less than 1% of patients on Axiron reported experiencing mastalgia and higher blood testosterone levels. The effects of testosterone on gender are typically reversible. Endogenous testosterone release is suppressed during exogenous androgen therapy through feedback suppression of pituitary luteinizing hormone (LH). Spermatogenesis inhibition may happen at high exogenous androgen concentrations due to feedback suppression of pituitary follicle stimulating hormone (FSH). Similar to other testosterone therapies, decreased serum testosterone and oligospermia have been reported during post approval surveillance of testosterone topical gel.
Both an increase and a decrease in libido may result with testosterone therapy. In clinical evaluation of testosterone gel (Androgel), libido decrease was reported in 1—3% of patients. Males in their senior years are more likely to experience priapism and excessive sexual excitement as a result of taking too much testosterone. One percent of individuals receiving Testim 50 or 100 mg daily reported experiencing spontaneous penile erections. Priapism and impotence (erectile dysfunction) were documented after post-approval experience with testosterone topical gel (Fortesta).
Long-term testosterone therapy can cause prostatic enlargement, and older male patients are more likely to have these side effects. One percent of 205 patients using the testosterone gel Testim 50 or 100 mg daily were found to have benign prostatic hyperplasia, or BPH. Clinical trials for testosterone patch (Androderm) include reports of unspecified prostate abnormalities in 5% of patients. Less than 1% of people on Axiron had a recorded case of prostate cancer. In clinical trials for testosterone topical solution (Axiron: 1–4 percent), topical gel (Fortesta: 1.3 percent), and intranasal gel (Natesto: 5.1–5.8 percent), elevations in serum PSA concentrations have also been noted. Prostate disorders (3–5%) including enlarged prostate, BPH, and increased PSA were reported in a 180-day Phase 3 study of testosterone gel (Androgel), as were testis disorders (1.9–3%) including left varicocele and mild testicular sensitivity. Approximately 18% of patients (n = 29) in a 3-year open-label extension experiment including 162 hypogonadal males taking testosterone gel (Androgel) saw increases in blood PSA concentrations (defined as >= 2x baseline values or any single absolute value >= 6 ng/ml). The majority of these increases (23/29 or 79%) were noticed in the first year of therapy. A single value >= 6 ng/ml was present in four patients, two of whom had prostate cancer discovered through biopsy. In the same study population, enlarged prostate and urinary symptoms including nocturia, urinary hesitancy, urinary incontinence, urinary retention, urinary urgency and weak urinary stream were also reported. Finally, 1 patient reported prostate disorder requiring a transurethral resection of the prostate (TURP) considered possibly related to treatment by investigators. Dysuria and hematuria have also been reported during postmarketing surveillance of testosterone therapy. Hematuria (< 3%), prostatitis (< 3%), and polyuria (< 3%) have been reported in patients receiving Androderm. In patients receiving testosterone therapy, surveillance for prostate cancer (as a secondary malignancy) should be consistent with current practices for eugonadal men. Signs of acute epididymitis (e.g., pyrexia, pain in the inguinal region) and/or urinary urgency should prompt withdrawal of the drug and reevaluation of dosage.
When androgens are administered to females, virilization—which can show as acne, the development of facial hair or an excessive amount of body hair (hirsutism), an enlarged clitoris, smaller breasts, and a deeper voice—can take place. When these symptoms first start, if testosterone therapy is stopped, they typically go away. Hirsutism was among the dermatological effects that were documented after approval or in less than 1% of individuals who used testosterone gel. Long-term treatment should be weighed against the risk because it can result in permanent masculinity. Amenorrhea or oligomenorrhea can result from a disruption of the regular menstrual cycle caused by testosterone-induced reduction of gonadotropin production. Testosterone is linked to teratogenesis and could harm an unborn child. A fetus’s (male or female) exposure to androgens may virilize them to varied degrees. The transmission of topical testosterone from male to female partners is one way that should be avoided during pregnancy.
Skin responses at the application site are linked to topical testosterone products. Transient mild to moderate erythema was seen at the site of application in the majority of individuals throughout therapy in clinical tests with the testosterone patch (Androderm). Application site reactions of any type were reported 28 percent of the time overall (10 subjects with 13 adverse reactions). Adverse reactions at the application site have been reported as pruritus (17–37%), burn-like blister reaction under the system (12%), erythema ( 7%), exfoliation ( 3%) and vesicular rash (6%) as well as allergic contact dermatitis to the system (4%) and burning (3%) and induration (3%) and a general rash (unspecified) (2%) Bullous rash, skin necrosis, or the emergence of a skin ulcer are occasionally associated with blisters that were recorded during trails. The majority of lesions were discovered in situations where the patch was applied on bony prominences or over areas of the body that may have experienced prolonged pressure while lying or sitting. Other dermatological reactions at the application site that happen in less than 1% of patients include contamination, mechanical irritation, bullous rash, and rash (unspecified). 5% of patients stopped receiving treatment because of chronic skin discomfort. After removing the transdermal device, the afflicted area can be treated with an over-the-counter topical hydrocortisone lotion to reduce mild skin irritation. Additionally, it has been demonstrated that the occurrence and degree of skin irritation can be decreased by sparingly applying 0.1 percent triamcinolone acetonide cream to the skin beneath the transdermal system’s core drug reservoir. Triamcinolone ointment formulations shouldn’t be used for pretreatment as they may drastically impair testosterone absorption. However, the administration of 0.1 percent triamcinolone acetonide cream does not significantly modify transdermal absorption of testosterone from the system. Dermatological responses during testosterone topical solution (Axiron) clinical studies include erythema (5–7%), folliculitis ( 1%), and application site skin irritation (7–8%). Other less common adverse reactions include: general erythema (< 1%) and application site edema and warmth (reported in at least 2 patients). Additionally, testosterone gel application site reactions have been documented (Fortesta: 16.1%; Androgel: 3–5.6%; Testim: 2–4%). In addition to xerosis (1.9%), acne (1–8%), and pruritis, testosterone gel (Androgel) has also been associated with other dermatological side effects during clinical trials (1.9 percent ). In 2.1% of individuals who used testosterone gel, contact dermatitis was noted (Androgel 1.62 percent ). All testosterone therapy influences the growth and secretion of the sebaceous glands, which can cause seborrhea and acne indistinguishable from acne vulgaris. Acne vulgaris (> 1%) was reported in a clinical evaluation of testosterone solution (Axiron). Patients undergoing protracted therapy or high dosages of testosterone have also developed alopecia that resembles male pattern baldness. Any brand of testosterone gel has been associated with the following dermatological side effects that have been reported after approval or in less than 1% of patients using the product: acne, allergic dermatitis, diaphoresis, alopecia, erythema, hair discoloration, maculopapular rash, paresthesias, pruritus, rash (unspecified), skin irritation, swelling, and xerosis. Patients taking testosterone undecanoate were shown to have hyperhidrosis (1.3%) during clinical evaluation and post-marketing surveillance.
The testosterone buccal mucoadhesive system has been linked to dental pain, including gum or mouth irritation (9.2%), dysgeusia (4.1%), gum pain (3.1%), gum soreness (3.1%), gum edema (2%) and taste distortion (dysgeusia, 2 percent ). The majority of gum-related adverse events were brief; gum soreness and irritation typically subsided within 1–8 days and 1–14 days, respectively. During clinical trials, 1 patient experienced buccal mucosal roughening, gingivitis, gum blisters, nose edema, lip stinging, and toothache. In clinical trials, adverse events involving the mouth or gums led to treatment discontinuation in 4.1% of participants. In one study, gum exams were done to check for gingivitis, gum edema, oral lesions, oral ulceration, or leukoplakia. None of these anomalies were found, and none of them had any new or worsening instances. The following side events—buccal inflammation, xerostomia, gum redness, stomatitis, taste bitter/taste perversion (dysgeusia), and toothache—occurred in 1 patient each in 2 long-term extension trials. Dysgeusia (reported as taste disorder) was reported in 1% of patients receiving testosterone gel (Testim) and judged possibly, probably, or definitely related to the study drug. However, other topical or injectable testosterone medications have not been associated with dysgeusia as a side effect, and systemic and topically applied testosterone are not known to frequently induce taste disruption.
In pre-pubertal males, early exposure to pharmaceutical amounts of testosterone or other androgens can cause virilism, which can be harmful because it is accompanied by an early closure of the epiphysis. Growth stops once the epiphyses have closed. Monitoring the development of the skeleton should be done every six months or so. Growth stops once the epiphyses have closed. Even after stopping testosterone therapy, epiphyseal closure can still be improved for a few months.
Retention of salt, chloride, water, potassium, and inorganic phosphates has been linked to androgen therapy. Weight gain may be a symptom of peripheral edema, which can develop as a result of increased fluid retention in conjunction with sodium chloride. These effects could be more noticeable in the beginning of androgen therapy. Only a minor degree of fluid retention happens when hypogonadism is treated with standard therapeutic testosterone levels. The fluid retention is more important when treating individuals with congestive heart failure or poor renal function. In animal models, testosterone has been shown to elevate blood pressure, change naturesis, cause vasoconstriction, and stimulate the renin-angiotensin-aldosterone pathway. As a result, androgens may alter blood pressure; nevertheless, it is still unknown how testosterone regulates blood pressure. During the clinical evaluation and post-approval monitoring of testosterone therapy, hypertension has been noted. 2.1–3% of patients taking testosterone gel (Androgel) in clinical studies complained of hypertension. Hypertension (1%) as well as decreased diastolic pressure (1%) were reported in trials involving testosterone gel (Testim). Hypertension (>1%) was reported in patients using testosterone topical solution (Axiron). Androgens may influence the prevalence of cardiovascular disease in addition to blood pressure. Investigations are now being conducted to determine whether testosterone usage, regardless of pre-existing cardiac illness, is associated with an increased risk of serious cardiovascular events. Adult male patients with an average age of 60 were included in an observational study at the U.S. Veterans Affairs medical system. Retrospective analysis comprised patients (n = 8779) undergoing coronary angiography with a documented low blood testosterone levels of less than 300 ng/dl. Within the larger group, 1223 guys underwent testosterone therapy after a median of 531 days had passed since coronary angiography; 7486 males did not. In the three years following coronary angiography, individuals taking testosterone therapy experienced a serious and/or fatal cardiovascular event at a rate of 25,7% compared to patients not receiving therapy at a rate of 19,9%. (myocardial infarction, stroke, death). The incidence of acute non-fatal myocardial infarction (MI) after a first prescription for testosterone was examined in a second observational research (n = 55,593) in both younger (=55 years) and older (>=65 years) adult males. When compared to the incidence rate of MI happening in the year preceding the first testosterone prescription, the incidence rate of MI occurring 90 days after the first prescription was studied. There was a 2-fold increase in MI risk among older men during the 90-day window, and there was a 2- to 3-fold increase in MI risk among younger men with a history of cardiac disease. Younger men without a history of heart disease, in comparison, did not exhibit an elevated risk. In light of these findings, the FDA announced in early 2014 an examination into the possible link between testosterone therapy and severe cardiovascular events. The FDA has NOT concluded that FDA-approved testosterone treatment increases the risk of stroke, MI, or death. However, health care professionals are urged to carefully consider whether the benefits of treatment are likely to exceed the potential risks. The FDA will communicate their final conclusions and recommendations when the evaluation is complete.
Certain androgens can cause hepatic dysfunction, hence it is advisable to monitor liver function tests on a regular basis.As opposed to overt jaundice or other liver problems, which are uncommon with testosterone use in general, increased hepatic enzymes are more likely to occur with use as advised. Administration of 17-alpha-alkylating hormones (like methyltestosterone) or abuse of such androgenic hormones by athletes are more likely to have negative effects on the liver. Abuse of these hormones causes liver changes consistent with fatty liver disease (steatosis) in an estimated 2.4 percent of people, even in the absence of other risk factors for fatty liver. If cholestatic jaundice, hepatitis, or another serious liver malfunction occurs, testosterone should be stopped. Hepatic neoplasms and periosis hepatis are uncommon, but when they do develop, they can be fatal.
Numerous testosterone therapy trials have observed headache; regardless of formulation, incidence rates of headache range from 1 to 6 percent. A few instances of mood changes, such as emotional lability ( 3%), bewilderment ( 1%), despair ( 3%), nervousness ( 3%), anxiety (> 1%), rage (> 1%), asthenia ( 1%), hostility ( 1%), and mood swings ( 1%), have also been noted in several testosterone investigations. In patients using testosterone gel, abnormal dreams (Fortesta: 1.3%) and insomnia (Testim: 1%) have also been seen. For patients receiving testosterone, hot flashes or flushing were also documented (Testim: 1%; Androgel: 1%–3%). Patients using testosterone solution have had vomiting (3–4%) and diarrhea (3–4%). (Axiron). Diarrhea (< 3%), gastroesophageal reflux disease (< 3%), back pain (6%), chills (< 3%), fatigue (< 3%) have been reported in patients receiving Androderm transdermal patch. Regardless of formulation, other side effects associated with exogenous testosterone have been reported post-approval or in less than 1% of patients who used it. These include abdominal pain (cramps), abnormal renal function, appetite stimulation, asthma, dizziness, hyperglycemia, increased lacrimation, malaise, nausea, pain in extremity (musculoskeletal pain), pelvic pain, and vitreous detachment. Other unrelated side effects of testosterone undecenoate that have been documented during post-approval monitoring include myalgia, tinnitus, and abrupt hearing loss.
Osteolysis has been brought on by testosterone medication, and it can make hypercalcemia worse. Particularly in sedentary patients and those with metastatic breast cancer, androgen-induced hypercalcemia might arise. Osteopenia and osteoporosis were identified as skeletal adverse effects during post-approval surveillance of testosterone undecanoate.
Hypercholesterolemia and hypertriglyceridemia are two unfavorable alterations in serum lipid profiles that can be brought on by testosterone. Throughout treatment, regular lipid profile monitoring may be desirable.1 The lipid profiles of post-menopausal women, bodybuilders, and weightlifters who use anabolic steroids have been found to shift in a “pro-atherogenic” way, with lower HDL levels and higher LDL levels. The HDL-C:LDL-C ratio may be decreased by synthetic androgens more than testosterone. Although the effects of androgen-induced hypercholesterolemia are not yet understood, patients who are prone to dyslipidemia or atherosclerosis should be especially cautious. If lipid changes are considerable, testosterone or lipid-lowering medication dosage adjustments or testosterone treatment termination may be necessary; individualize therapy.
The synthesis of erythropoietin is stimulated by testosterone. Increased erythropoiesis, particularly in females, can result in secondary polycythemia and accompanying consequences, such as erythrocytosis, which can cause headaches, weariness, dizziness, and irregular bleeding as well as skin redness and flushing. Polycythemia is a danger for patients taking large doses of testosterone. Increases in red blood cell count ( 1%), hematocrit (4–7%%), and hemoglobin (> 1%) were observed in clinical tests with testosterone solution (Axiron). In studies of testosterone gel (Testim), patients receiving a 100 mg dose had clinically notable increases in both hematocrit (2.8%) and hemoglobin (2.3%). The hematocrit or hemoglobin levels of 2.1% of patients receiving testosterone gel (Androgel 1.62%) were also higher. In an intranasal testosterone gel investigation, 4 of 306 exposed patients had hematocrit levels that were higher than 55% (baseline: 48–51%; did not go over 58%). Therefore, periodic hemoglobin and hematocrit determinations should be considered in patients receiving long-term testosterone therapy. In general, testosterone therapy has been associated with suppression of clotting factors II, V, VII, and X and bleeding in patients on concomitant anticoagulant therapy.7 GI bleeding was reported in 2% of patients receiving testosterone patch (Androderm) therapy during clinical evaluation. Hemarthrosis (< 3%) has also been reported Androderm. During postmarketing surveillance of testosterone gel (Testim), prolonged aPPT and PT and prolonged bleeding time were reported. Anemia was reported in 2.5% of patients receiving testosterone gel (Androgel) during clinical evaluation. Use of testosterone is linked to an elevated risk of deep vein thrombosis (DVT) and acute pulmonary embolism (PE); incidents have been recorded during post-marketing surveillance. In individuals displaying leg discomfort, warmth, swelling, and redness (DVT) or chest pain, wheezing, and coughing (PE), stop testosterone therapy and check for potential VTE. Other miscellaneous reactions reported during post approval surveillance of testosterone undecenoate include: thrombocytopenia, hyperparathyroidism, and hypoglycemia.
Anabolic steroids used intramuscularly may result in erythema, urticaria, post-injection discomfort, induration, and furunculosis. At the location of the testosterone implant pellets’ implantation, inflammation and pain are likely. Additionally, testosterone pellets may slough off from the site of insertion. This usually happens as a result of shallow implantation or aseptic approach. You should keep an eye on the patients for any indications of an injection site reaction.
There haven’t been many examples of anaphylactoid responses linked to oral and injectable testosterone therapy. The use of testosterone undecanoate has been linked to anaphylactic and significant pulmonary oil microembolism (POME) reactions as well as pulmonary embolism instances. During or right away following a 1000 mg intramuscular injection of testosterone undecanoate, incidences of POME responses have been reported. Coughing, the desire to cough, dyspnea, hyperhidrosis, throat constriction (acute bronchospasm), chest pain, lightheadedness, and syncope were among the symptoms. However, some cases prolonged up to several hours and required emergency care and/or hospitalization. The majority of cases lasted a few minutes and were treated with supportive measures. In addition to POME reactions, instances of anaphylaxis, including potentially fatal events, have also been documented after testosterone undecanoate injection intramuscularly. In total, 18 clinical trials recorded 9 POME incidents in 8 people and 2 anaphylactic events among 3556 patients receiving testosterone undecanoate; incidences of both POME and anaphylaxis were also reported post-approval. In the course of the usual course of treatment, cases have happened both after the initial injection and during subsequent injections. Monitor the patient for 30 minutes following each administration, and in the event of severe POME or anaphylactoid reactions, administer the necessary medical care.1 Due to the risk of serious POME and anaphylaxis reactions, testosterone undecanoate (Aveed) is only available through a restricted program called the Aveed REMS Program. Clinicians wanting to prescribe Aveed, must be certified with the REMS Program for purposes of ordering or dispensing the product. Healthcare settings must also be certified with the REMS Program and must have the resources to provide emergency medical treatment in cases of serious POME and anaphylaxis. Further information is available at www.AveedREMS.com or call 1—855—755—0494. Transient respiratory reactions including the urge to cough, coughing fits, and respiratory distress immediately after intramuscular injection of testosterone enanthate have been reported during post-marketing surveillance. Care should be taken to ensure slow and deep gluteal muscle injection of testosterone preparations. Nasopharyngitis or pharyngitis (> 1 %) was reported in patients receiving testosterone topical solution (Axiron).
In individuals with sleep apnea risk factors, such as obesity or chronic lung disease, testosterone therapy for hypogonadal males may raise the risk of developing sleep apnea.fn]Androgel (testosterone gel) package insert. Marietta, GA: Solvay Pharmaceuticals, Inc.; 2012 Sept.
The following nasal side effects were among the most frequent adverse events in clinical studies of intranasal testosterone gel: nasopharyngitis (3.8–8.7% percent), rhinorrhea (3.8–7.8%), parosmia (5.8%), epistaxis (3.8–6.5%), nasal irritation or discomfort (3.8–5.9 percent), nasal scabbing (3.8–5.8 percent), nasal dryness (4.2 percent), nasal congestion (3.9 percent), and procedural pain (4.3 percent ). Long-term data on nasal safety are scarce, despite the fact that the majority of nasal complaints were mild or moderate in severity. Encourage patients to disclose any uncomfortable nose symptoms; if any exist, decide if they call for additional testing or ongoing care. Other respiratory side effects include sinusitis (3.8–4.3%), bronchitis (3.8–4.3%), and upper respiratory tract infections (3.8–4.3%). (3.8 percent ).
If you experience any of the following symptoms of an allergic reaction, call your doctor right away: skin rash, itching or hives, swelling of the face, lips, or tongue; a blue tint to the skin; tightness in the chest; pain; difficulty breathing; wheezing; dizziness; or a red, swollen, painful area on the leg.
Store this medication at 68°F to 77°F (20°C to 25°C) and away from heat, moisture and light. Keep all medicine out of the reach of children. Throw away any unused medicine after the beyond use date. Do not flush unused medications or pour down a sink or drain.
Learn how to prepare medication for self-administered injection.
1.Aveed (testosterone undecanoate Injection) package insert. Malvern, PA: Endo Pharmaceuticals Solutions Inc.; 2014 Mar.
2.DELATESTRYL (Testosterone Enanthate Injection, USP) package insert. Lexington, MA: Indevus Pharmaceuticals, Inc.; 2007 July.
3.Axiron (testosterone) topical solution, package insert. Indianapolis, IN: Lilly USA, LLC; 2011 Dec.
4.The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60:616-31.
5.Vigen R, O’Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
6.WD Finkle, S Greenland, GK Ridgeway, et al. Increased Risk of Non-Fatal Myocardial Infarction Following Testosterone Therapy Prescription in Men. DOI: 10.1371/journal.pone.0085805
7.FDA Medwatch – FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. Retrieved January 31, 2014. Available on the World Wide Web http://www.fda.gov/Drugs/DrugSafety/ucm383904.htm
8.Natesto (testosterone) nasal gel package insert. Durants, Christ Church Barbados: Trimel BioPharma SRL; 2014 May.
9.Testim (testosterone gel) package insert. Malvern, PA: Auxilium Pharmaceuticals, Inc.; 2010 Apr.
10.Androderm (testosterone transdermal system) package insert. Corona, CA: Watson Pharma, Inc.; 2014 Jun.
11.Androgel (testosterone gel) package insert. Marietta, GA: Solvay Pharmaceuticals, Inc.; 2012 Sept.
12.Striant (testosterone buccal system) package insert. Livingston, NJ: Columbia Laboratories, Inc.; 2014 Mar.
13.Fortesta (testosterone) gel, package insert. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010 Dec.
14.DEPO-TESTOSTERONE (testosterone cypionate) injection, package insert. New York, NY: Pharmacia & Upjohn Co.; 2006 Sept.
15.Kochenour NK. Lactation suppression. Clin Obstet Gynecol. 1980;23:1045-1059.
16.Krauser JA, Guengerich FP. Cytochrome P450 3A4-catalyzed testosterone 6beta-hydroxylation stereochemistry, kinetic deuterium isotope effects, and rate-limiting steps. J Biol Chem 2005;280:19496—506.
17.Barnes KM, Dickstein B, Cutler GB Jr, et al. Steroid transport, accumulation, and antagonism of P-glycoprotein in multidrug-resistant cells. Biochemistry 1996;35:4820—7.
18.Wells PS, Holbrook AM, Crowther NR et al. Interaction of warfarin with drugs and food. Ann Intern Med 1994;121:676—83.
19.Goffin E, Pirson Y, Geubel A, et al. Cyclosporine-methyltestosterone interaction. Nephron 1991;59:174—5.
20.Borras-Blasco J, Rosique-Robles JD, Peris-Marti J, et al. Possible cyclosporin-danazol interaction in a patient with aplastic anaemia. Am J Hematol 1999;62:63—4.
21.Moller BB, Ekelund B. Toxicity of cyclosporine during treatment with androgens. N Engl J Med 1985;313:1416.
22.Ross WB, Roberts D, Griffin PJ, et al. Cyclosporin interaction with danazol and norethisterone. Lancet 1986;1:330.
23.Androgel® (testosterone gel) package insert. Montrogue, France: Laboratories Besins International; 2005 Aug.
24.Zoladex® (goserelin acetate) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2003 Dec.
25.Viadur® (leuprolide implant) package insert. Westhaven, CT: Bayer Pharmaceuticals; 2002 May.
26.Humatrope™ (somatropin);package insert. Indianapolis, IN: Eli Lilly and Company; 2003 Jul.
27.Androderm® (testosterone transdermal system) package insert. Corona, CA: Watson Pharma, Inc.; 1999 Jan.
28.Propecia® (finasteride) package insert. Whitehouse Station, NJ: Merck & Co., INC.; 2003 Oct.
29.Avodart™ (dutasteride) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2005 May.
30.Robbers JE, Tyler VE. Tyler’s Herbs of Choice: the Therapeutic Use of Phytomedicinals. Binghamton NY: Haworth Herbal Press, Inc.; 1999.
31.German Commission E. Saw Palmetto berry, Sabal fructus, monograph Published March 2, 1989 and revised January 17, 1991. In: Blumenthal, M et al ., eds. The complete German Commission E Monographs -Therapeutic Guide to Alternative Medicines. Bosto
32.Lazar JD, Wilner KD. Drug interactions with fluconazole. Rev Infect Dis 1990;12:S327—33.
33.Hansten P, Horn J. The Top 100 Drug Interactions: A Guide to Patient Management. includes table of CYP450 and drug transporter substrates and modifiers (appendices). H & H Publications, LLP 2014 edition.
34.Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity, and partial androgen deficiency. Aging Male 2003;6:1—7.
35.Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity, and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Clin Endocrinol 2006; 154:899—90
36.Hobbs CJ, Jones RE, Plymate SR. Nandrolone, a 19-nortestosterone, enhances insulin-independent glucose uptake in normal men. J Clin Endocrinol Metab 1996; 81:1582—5.
37.Corrales JJ, Burgo RM, Garcia-Berrocal B, et al. Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism 2004;53:666—72
38.Lee CH, Kuo SW, Hung YJ, et al. The effect of testosterone supplement on insulin sensitivity, glucose effectiveness, and acute insulin response after glucose load in male type 2 diabetics. Endocrine Res 2005;31:139—148.
39.Cohen JC, Hickman R. Insulin resistance and diminished glucose tolerance in powerlifters ingesting anabolic steroids. J Clin Endocrinol Metab 1987;64:960—3.
40.Aldercreutz H, Mazur W. Phyto-estrogens and western diseases. Annals of Medicine 1997;29:95—120.
41.Ranexa (ranolazine extended-release tablets) package insert. Foster City, CA: Gilead Sciences, Inc. 2013 Dec.
42.Letairis™ (ambrisentan) package insert. Foster City, CA: Gilead Sciences, Inc; 2008 Oct.
43.Pradaxa (dabigatran) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015 Jan.
44.Gilotrif (afatinib) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2013 Nov.
45.Androgel 1.62% (testosterone gel) package insert. North Chicago, IL: Abbott Laboratories; 2014 Nov.
46.Naik BS, Shetty N, Maben EVS. Drug-induced taste disorders. European Journal of Internal Medicine 2010; 21:240-243.
Be the first to review “Testosterone Cypionate / Testosterone Propionate Injection”
General Inquiries
There are no inquiries yet.








Reviews
There are no reviews yet.