- Description
- Reviews (0)
- Store Policies
- Inquiries
Description
Overview of Phentermine HCl Capsules
-
Dosage Strengths of Phentermine HCl Capsules/Tablets
- Commercial: 30 mg Capsule
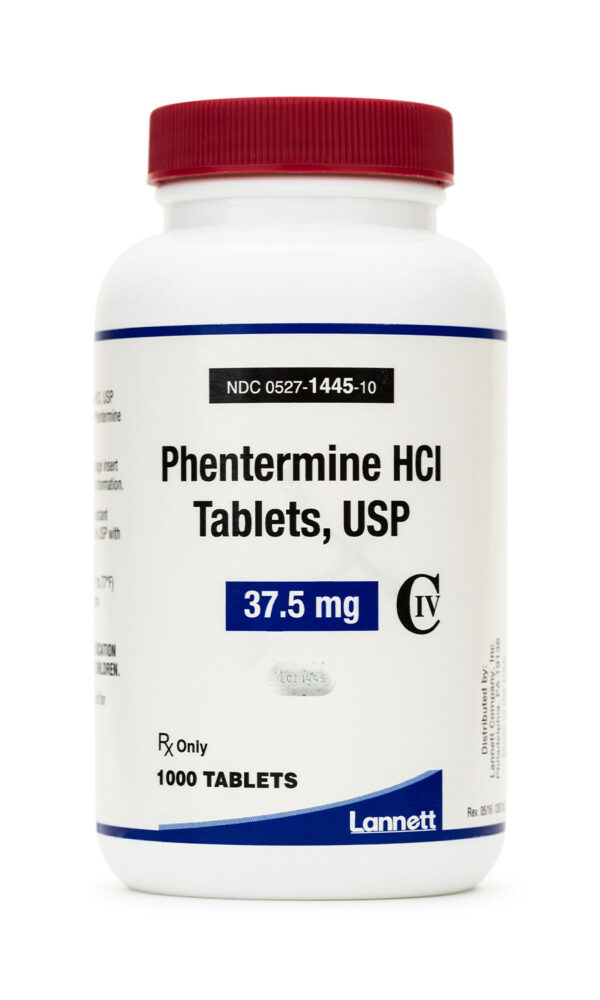
Commercial: 37.5 mg Tablet
Compounded: 45 mg Capsule
Compounded: 37.5 mg Slow-Release Capsule
Compounded: 45 mg Slow-Release Capsule
Phentermine is an oral sympathomimetic amine used as an adjunct for short-term (e.g., 8—12 weeks) treatment of exogenous obesity. The pharmacologic effects of phentermine are similar to amphetamines. Phentermine resin complex was approved by the FDA in 1959, but is no longer marketed in the US. Phentermine hydrochloride was FDA approved in 1973. In the mid-90s, there was renewed interest in phentermine in combination with another anorectic, fenfluramine, for the treatment of obesity and substance abuse, however, little scientific data support this practice. On July 8, 1997, the FDA issued a ‘Dear Health Care Professional’ letter warning physicians about the development of valvular heart disease and pulmonary hypertension in women receiving the combination of fenfluramine and phentermine; fenfluramine was subsequently withdrawn from the US market in fall of 1997. Use of phentermine with other anorectic agents for obesity has not been evaluated and is not recommended. In May 2011, the FDA approved a phentermine hydrochloride orally disintegrating tablet (Suprenza) for the treatment of exogenous obesity.
Limited data are available in reference texts regarding the mechanism of action of this drug. Phentermine is an analog of methamphetamine. Similar to the amphetamines, phentermine increases the release of norepinephrine and dopamine from nerve terminals and inhibits their reuptake. Thus, phentermine is classified as an indirect sympathomimetic. Other effects include a weak ability to dose-dependently raise serotonin levels, although the effect on serotonin occurs is less potent than that of methamphetamine itself. Clinical effects include CNS stimulation and elevation of blood pressure. Appetite suppression is believed to occur through direct stimulation of the satiety center in the hypothalamic and limbic region.
Tolerance to the anorexiant effects of phentermine usually develops within a few weeks of starting therapy. The mechanism of tolerance appears to be pharmacodynamic in nature; higher doses of phentermine are required to produce the same response. When tolerance develops to the anorexiant effects, it is generally recommended that phentermine be discontinued rather than the dose increased.
Phentermine is administered orally. The rate and extent of phentermine exposure under fasting conditions is equivalent regardless of oral formulation administered.
Limited data exist on the pharmacokinetics of phentermine. Phentermine is primarily excreted by the kidneys. The elimination half-life ranges 19—24 hours and is influenced by urinary pH. Because the pKa of phentermine is 9.84, the elimination half-life decreases to about 7—8 hours under acidic urinary conditions.
Route-Specific Pharmacokinetics:
Oral Route: Following oral administration, most absorption of phentermine occurs from the small intestine. The duration of action following administration of the 8 mg capsules or tablets is about 4 hours and 12—14 hours after administration of the 30 mg capsules or the 37.5 mg tablets.
Phentermine oral disintegrating tablet (ODT) reaches peak concentrations (Cmax) 3—4.4 hours post-administration. Water ingestion prior to swallowing the ODT did not affect the AUC. Despite a decrease in the Cmax (approximately 5%) and AUC (approximately 12%) when phentermine ODT was administered after a high fat/high calorie breakfast, phentermine ODT can be administered with or without food. The Cmax and AUC were decreased by approximately 7% and 8%, respectively, when the ODT was swallowed without prior disintegration.
Special Populations:
Renal Impairment: Use with caution in patients with renal impairment. Cumulative urinary excretion of phentermine under uncontrolled urinary pH conditions is 62—85%, and exposure increases can be expected in patients with renal impairment.
Phentermine is contraindicated for use in any patient with a prior history of sympathomimetic amine hypersensitivity.
According to the manufactures of phentermine capsules and tablets, its products are contraindicated in patients with cardiac disease, advanced arteriosclerosis, moderate to severe hypertension, agitated states, or glaucoma. Likewise, orally disintegrating tablets, are contraindicated in patients with a history of cardiac disease including coronary artery disease, stroke, cardiac arrhythmias, heart failure, and uncontrolled hypertension. Valvular heart disease has been reported in women receiving the combination of fenfluramine and phentermine; the safety and efficacy of combination therapy with phentermine and any other drug products for weight loss, including selective serotonin reuptake inhibitors (e.g., fluoxetine, sertraline, fluvoxamine, paroxetine), have not been established. Therefore, coadministration of these drug products for weight loss is not recommended. Further, primary pulmonary hypertension (PPH) has been reported to occur in patients receiving a combination of phentermine with fenfluramine or dexfenfluramine. The possibility of an association between the use of phentermine alone and PPH or valvular heart disease cannot be ruled out. The initial symptom of PPH is usually dyspnea. Other initial symptoms include: angina pectoris, syncope, or lower extremity edema. Patients should be advised to report immediately any deterioration in exercise tolerance. Treatment should be discontinued in patients who develop new, unexplained symptoms of dyspnea, angina pectoris, syncope, or lower extremity edema.
Because phentermine is a sympathomimetic agent, it is contraindicated in patients with hyperthyroidism. It should also be used with caution in patients with thyroid disease.
Phentermine is contraindicated for use during or within 14 days following the use of MAOI therapy or other drugs with MAO-inhibiting activity. Monoamine oxidase inhibitors (MAOIs), or drugs that possess MAO-inhibiting activity such as furazolidone or procarbazine, can prolong and intensify the cardiac stimulation and vasopressor effects of phentermine.
Phentermine is contraindicated in patients with agitated states.aggravate these effects or cause an adverse drug reaction. Symptoms of chronic intoxication include insomnia, irritability, change in personality, and psychotic symptoms that may be clinically indistinguishable from other psychotic disorders, like schizophrenia. Phentermine could aggravate certain mental conditions, such as those patients who exhibit highly nervous or agitated behavior, including psychosis, mania, or severe anxiety.
The use of phentermine may cause dizziness, mask signs of fatigue or the need for rest, or impair the ability of a patient to participate in activities that require mental alertness. Advise patients to use caution when driving or operating machinery, or performing other tasks that require mental alertness until they are aware of how therapy will affect their mental and/or motor performance. In general, ethanol ingestion may aggravate these effects or cause an adverse drug reaction. Advise patients to avoid alcohol while taking phentermine.
Use phentermine cautiously in patients with diabetes mellitus. Insulin or other antidiabetic medication requirements may be altered in these patients when using phentermine during weight loss and due to altered dietary regimens. Patients should monitor their blood glucose regularly and follow the recommendations of their health care provider.
Appetite suppressant therapy is not recommend for use in those patients with a history of anorexia nervosa or other eating disorders. Use of phentermine is contraindicated in patients with a known history of drug or substance abuse. Phentermine is chemically and pharmacologically related to the amphetamines which have been extensively abused. The possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. The least amount reasonable should be prescribed or dispensed at one time in order to limit the potential for overuse or drug diversion.
Phentermine products are now classified as FDA pregnancy risk category X, as are many anorexiants used for weight loss, and are contraindicated during pregnancy. Safe use of phentermine during pregnancy has not been established; there is no known indication for use of phentermine during pregnancy. Phentermine should not be taken by pregnant women or by women who may become pregnant unless, in the opinion of the physician, the potential benefits outweigh the possible hazards.
Abrupt discontinuation of phentermine after prolonged high doses may result in severe mental depression or extreme fatigue; sleep EEG changes have also been noted. Gradual withdrawal of therapy is recommended. If immediate discontinuation is medically necessary, careful monitoring and symptom management is warranted.
Phentermine is contraindicated during breastfeeding. It is not known whether phentermine and its metabolites are excreted in breast milk; however, because of the potential for serious adverse effects in the nursing infants, breastfeeding while taking phentermine is not recommended.
Safety and effectiveness of phentermine in children have not been established. Phentermine is not recommended for children or adolescents 16 years of age and under. There is no established use of phentermine in infants or neonates.
The debilitated or geriatric patient may be more susceptible to the CNS and sympathomimetic side effects of phentermine; use with caution in elderly patients. Patients with renal impairment may also be more susceptible to side effects. Exposure increases can be expected in patients with renal impairment or renal failure. Use caution when administering phentermine to patients with renal impairment.
The use of inhalational anesthetics during surgery may sensitize the myocardium to the effects of sympathomimetic drugs. Because of this, and its effects on blood pressure, in general, phentermine should be discontinued several days prior to surgery. Avoid abrupt discontinuation.
Phentermine products are now classified as FDA pregnancy risk category X, as are many anorexiants used for weight loss, and are contraindicated during pregnancy. Safe use of phentermine during pregnancy has not been established; there is no known indication for use of phentermine during pregnancy. Phentermine should not be taken by pregnant women or by women who may become pregnant unless, in the opinion of the physician, the potential benefits outweigh the possible hazards.
Phentermine is contraindicated during breastfeeding. It is not known whether phentermine and its metabolites are excreted in breast milk; however, because of the potential for serious adverse effects in the nursing infants, breastfeeding while taking phentermine is not recommended.
The safety of phentermine when used with other anorexiant agents such as amphetamine, benzphetamine, dexfenfluramine, dextroamphetamine, diethylpropion, ephedrine, fenfluramine, and sibutramine is controversial and concurrent use should be avoided. The role of phentermine in the production of cardiac valvulopathy when combined with dexfenfluramine, fenfluramine, or other medications for weight loss is uncertain. The combined use of these agents may have the potential for additive side effects, such as hypertensive crisis or cardiac arrhythmias. Similarly, because phentermine is a sympathomimetic and anorexic agent (i.e., psychostimulant) it should not be used in combination with other sympathomimetics or psychostimulants for weight loss, including OTC preparations, and herbal products that may contain ephedra alkaloids or Ma huang.
Phentermine, which increases catecholamine release, can increase blood pressure; this effect may be additive with the prolonged vasoconstriction caused by ergot alkaloids. Monitoring for cardiac effects during concurrent use of ergot alkaloids with phentermine may be advisable.
Concurrent use of bromocriptine and some sympathomimetics such as phentermine should be approached with caution. One case report documented worsening headache, hypertension, premature ventricular complexes, and ventricular tachycardia in a post-partum patient receiving bromocriptine for lactation suppression who was subsequently prescribed acetaminophen; dichloralphenazone; isometheptene for a headache. A second case involved a post-partum patient receiving bromocriptine who was later prescribed phenylpropanolamine; guaifenesin and subsequently developed hypertension, tachycardia, seizures, and cerebral vasospasm.
In theory, an interaction is possible between cabergoline, an ergot derivative, and some sympathomimetic agents such as phentermine. Use of the ergot derivative bromocriptine for lactation suppression in conjunction with a sympathomimetic (i.e., isometheptene or phenylpropanolamine) for other therapeutic uses has resulted in adverse effects such as worsening headache, hypertension, ventricular tachycardia, seizures, sudden loss of vision, and cerebral vasospasm.
Concurrent use of dronabinol, THC or nabilone with sympathomimetics may result in additive hypertension, tachycardia, and possibly cardiotoxicity.
Monoamine oxidase inhibitors (MAOIs), or drugs that possess MAO-inhibiting activity such as furazolidone, linezolid, or procarbazine, can prolong and intensify the cardiac stimulation and vasopressor effects of phentermine. Phenelzine and tranylcypromine appear to produce the greatest risk since these two MAOIs also have intrinsic amphetamine-like activity. In the presence of MAOIs, phentermine and other drugs that cause release of norepinephrine induce severe cardiovascular and cerebrovascular responses. It is unclear if selegiline, an inhibitor of MAO type B, can also predispose to this reaction. Phentermine should not be administered during or within 14 days following the use of most MAOIs or drugs with MAO-inhibiting activity. Rasagiline is a selective MAO-B inhibitor at manufacturer recommended doses; therefore, serious reactions with sympathomimetics are not ordinarily expected. However, because a case of elevated blood pressure occurred during use of rasagiline and a sympathomimetic ophthalmic preparation, caution is advised when rasagiline is administered with sympathomimetics.
The pressor response to some sympathomimetics is exaggerated in patients currently receiving tricyclic antidepressants. Concomitant use of tricyclic antidepressants with sympathomimetics, including phentermine, should be avoided whenever possible.
Phentermine has vasopressor effects and may limit the benefit of antihypertensive agents particularly sympatholytic agents such as guanadrel, guanethidine, methyldopa or reserpine. Phentermine may displace guanethidine from the neuron and antagonize the neuronal blockade caused by guanethidine. Concomitant use of phentermine with methyldopa or reserpine may antagonize the antihypertensive effects of these agents. Although leading drug interaction texts differ in the potential for an interaction between phentermine and this group of antihypertensive agents, these effects are likely to be clinically significant and have been described in hypertensive patients on these medications.
Use caution in combining phentermine with antidiabetic agents. Phentermine exhibits sympathomimetic activity. Sympathomimetics may increase blood sugar via stimulation of beta2-receptors which leads to increased glycogenolysis. A pharmacodynamic interaction with antidiabetic agents may occur. Diabetic patients may have decreased requirements of insulins, sulfonylureas, or other antidiabetic agents in association with the use of phentermine and the concomitant dietary regimen and weight loss. As long as blood glucose is carefully monitored to avoid hypoglycemia or hyperglycemia, it appears that phentermine can be used concurrently.
Halogenated anesthetics may sensitize the myocardium to the effects of the sympathomimetics. Because of this, and its effects on blood pressure, phentermine should be discontinued several days prior to surgery.
Concurrent use of phentermine and phenothiazines may antagonize the anorectic effects of phentermine. In addition, psychostimulants can aggravate psychotic states.
Although not studied, the concomitant use of ethanol and phentermine may result in an adverse reaction and should be avoided.
Phentermine, like other sympathomimetics, is contraindicated in selected patients with thyroid disease; caution should be used if coadministering thyroid hormones with phentermine.
Atomoxetine has been reported to increase blood pressure and heart rate, probably via inhibition of norepinephrine reuptake. Due to an additive pharmacodynamic effect, phentermine and atomoxetine should be used together cautiously, particular in patients with a history of cardiac disease. Consider monitoring heart rate and blood pressure at baseline and regularly throughout treatment if these agents must be used together.
Bupropion is associated with a dose-related risk of seizures. Excessive use of psychostimulants, such as phentermine or the combination of phentermine; topiramate, may be associated with an increased seizure risk; therefore, seizures may be more likely to occur in patients receiving these weight loss aides with bupropion or bupropion-containing combinations. Other side effects might also occur, such as dizziness, blood pressure changes, or other side effects. Patients should be closely monitored if this combination is necessary. Do not combine therapy with phentermine or phentermine-combinations and bupropion; naltrexone due to this risk and the duplication of therapy for weight loss.
Due to the pharmacology of salmeterol, caution and close observation should also be used when fluticasone; salmeterol is used concurrently with other adrenergic sympathomimetics, administered by any route, to avoid potential for increased cardiovascular effects based on the pharmacology of salmeterol.
Use phentermine and selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) together with caution; use together may be safe and efficacious for some patients based on available data, provided the patient is on a stable antidepressant regimen and receives close clinical monitoring. Regular appointments to assess the efficacy of the weight loss treatment, the emergence of adverse events, and blood pressure monitoring are recommended Watch for excessive serotonergic effects. Phentermine is related to the amphetamines, and there has been historical concern that phentermine might exhibit potential to cause serotonin syndrome or cardiovascular or pulmonary effects when combined with serotonergic agents. One case report has been received of adverse reactions with phentermine and fluoxetine. However, recent data suggest that phentermine’s effect on MAO inhibition and serotonin augmentation is minimal at therapeutic doses, and that phentermine does not additionally increase plasma serotonin levels when combined with other serotonergic agents. In large controlled clinical studies, patients were allowed to start therapy with phentermine or phentermine; topiramate extended-release for obesity along with their antidepressants (e.g., SSRIs or SNRIs, but not MAOIs or TCAs) as long as the antidepressant dose had been stable for at least 3 months prior to the initiation of phentermine, and the patient did not have suicidal ideation or more than 1 episode of major depression documented. In analyses of the results, therapy was generally well tolerated, especially at lower phentermine doses, based on discontinuation rates and reported adverse events. Because depression and obesity often coexist, the study data may be important to providing optimal co-therapies.
Use phentermine and vortioxetine together with caution; use together may be safe and efficacious for some patients based on available data, provided the patient is on a stable antidepressant regimen and receives close clinical monitoring. Regular appointments to assess the efficacy of the weight loss treatment, the emergence of adverse events, and blood pressure monitoring are recommended. Watch for excessive serotonergic effects. Phentermine is related to the amphetamines, and there has been historical concern that phentermine might exhibit potential to cause serotonin syndrome or cardiovascular or pulmonary effects when combined with serotonergic agents. One case report has been received of adverse reactions with phentermine and the antidepressant fluoxetine. However, recent data suggest that phentermine’s effect on MAO inhibition and serotonin augmentation is minimal at therapeutic doses, and that phentermine does not additionally increase plasma serotonin levels when combined with other serotonergic agents. In large controlled clinical studies, patients were allowed to start therapy with phentermine or phentermine; topiramate extended-release for obesity along with their antidepressants (e.g., SSRIs or SNRIs, but not MAOIs or TCAs) as long as the antidepressant dose had been stable for at least 3 months prior to the initiation of phentermine, and the patient did not have suicidal ideation or more than 1 episode of major depression documented. In analyses of the results, therapy was generally well tolerated, especially at lower phentermine doses, based on discontinuation rates and reported adverse events. Because depression and obesity often coexist, the study data may be important to providing optimal co-therapies.
This medicine may be habit-forming with long-term use. Check medicines with healthcare provider. This medicine may not mix well with other medicines. Limit caffeine (for example, tea, coffee, cola) and chocolate intake. Use with this medicine may cause nervousness, shakiness, and fast heartbeat. Use birth control that you can trust to prevent pregnancy while taking this medicine.
Primary pulmonary hypertension, a rare and serious lung disease, has developed in patients who received a combination of phentermine along with fenfluramine or dexfenfluramine. Phentermine may cause this lung disease. This medicine may be habit-forming; avoid long-term use. Tell healthcare provider if you have a history of drug or alcohol abuse. May cause serious heart-related side effects. Tell healthcare provider if you have any heart disease.
If you suspect an overdose, call your local poison control center or emergency department immediately. Signs of a life-threatening reaction. These include wheezing; chest tightness; fever; itching; bad cough; blue skin color; fits; or swelling of face, lips, tongue, or throat. Severe behavioral problems. Chest pain or pressure or fast heartbeat. Severe dizziness or passing out. Very nervous and excitable. Severe headache. Any rash. No improvement in condition or feeling worse.
Central nervous system adverse reactions that have been reported in patients receiving phentermine include dizziness, dysphoria, euphoria, headache, insomnia, overstimulation, restlessness, and tremor. Psychosis at recommended doses may occur rarely in some patients.
Primary pulmonary hypertension (PPH) and cardiac valvulopathy (regurgitant cardiac valvular disease) have been reported with phentermine. The initial symptom of PPH is usually dyspnea; other initial symptoms include: angina pectoris, syncope, or peripheral edema. Patients should be advised to report immediately any deterioration in exercise tolerance. Treatment should be discontinued in patients who develop new, unexplained symptoms of dyspnea, angina pectoris, syncope, or peripheral edema. Other cardiovascular adverse effects that have been reported include hypertension, ischemic events, palpitations, and sinus tachycardia.
Reported adverse gastrointestinal effects of phentermine include constipation, diarrhea, dysgeusia, nausea, and xerostomia.
Impotence (erectile dysfunction), libido increase, and libido decrease have been reported in patients receiving phentermine.
Urticaria has been reported in patients receiving phentermine.
Phentermine has not been systematically studied for its potential to produce dependence in obese patients treated with usual recommended dose ranges. Phentermine is related chemically and pharmacologically to the amphetamines, and these stimulant drugs have been extensively abused and the possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including this drug product as part of a weight reduction program. Abuse of amphetamines and related drugs (e.g., phentermine) may be associated with intense psychological dependence and severe social dysfunction. There are reports of patients who have increased the dosage of these drugs to many times than recommended. Physical dependence (physiological dependence) is a state that develops as a result of physiological adaptation in response to repeated drug use. Physical dependence manifests by drug-class-specific withdrawal symptoms after abrupt discontinuation or a significant dose reduction of a drug. Limited data are available for phentermine. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on a sleep electroencephalogram. Thus, in situations where rapid withdrawal is required, appropriate medical monitoring is recommended. Evidence-based data from the literature are relatively limited, and some experts suggest that long-term phentermine pharmacotherapy for obesity does not induce abuse or psychological dependence (addiction), drug craving, and that abrupt treatment cessation within the normal prescription dose range does not induce amphetamine-like withdrawal. More data are needed to confirm the dependence potential of phentermine-containing obesity products.
Tolerance to the anorexiant effects of phentermine usually develops within a few weeks of starting therapy. The mechanism of tolerance appears to be pharmacodynamic in nature; higher doses of phentermine are required to produce the same response. When tolerance develops to the anorexiant effects, it is generally recommended that phentermine be discontinued rather than the dose increased. The maximum recommended dose should not be exceeded.
Store this medication at 68°F to 77°F (20°C to 25°C) and away from heat, moisture and light. Keep all medicine out of the reach of children. Throw away any unused medicine after the beyond use date. Do not flush unused medications or pour down a sink or drain.
1.Suprenza (phentermine hydrochloride) package insert. Cranford, NJ: Akrimax Pharmaceuticals; 2011 Oct.
2.Adipex-P (phentermine hydrochloride tablets and capsules) package insert. Sellersville, PA: Teva Pharmaceuticals; 2013 Jan.
3.Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary disease. J Pharmacol Exp Ther. 2006;318:604-610.
4.Adipex-P (phentermine hydrochloride tablets and capsules) package insert. Sellersville, PA: Teva Pharmaceuticals; 2013 Jan.
5.Suprenza (phentermine hydrochloride) package insert. Cranford, NJ: Akrimax Pharmaceuticals; 2011 Oct.
6.Phentermine hydrochloride package insert. Newtown, PA: KVK-Tech Inc; 2010 April.
7.Steiner E, Villen T, Hallberg M, et al. Amphetamine secretion in breast milk. Eur J Clin Pharmacol 1984;27:123-4.
8.Meridia® (sibutramine) package insert. North Chicago, IL: Abbott Laboratories; 2003 Oct.
9.Fastin® (phentermine) package insert. Philadelphia, PA: Beecham Laboratories; 1987 Oct.
10.Dexedrine® (dextroamphetamine) package insert. Research Triangle Park, NC; GlaxoSmithKline; 2007 Mar.
11.Kulig K, Moore LL, Kirk M, et al. Bromocriptine-associated headache: possible life-threatening sympathomimetic interaction. Obstet Gynecol. 1991;78:941—3.
12.Cesamet™ (nabilone) package insert. Costa Mesa, CA: Valeant Pharmaceuticals International; 2006 May.
13.Foltin RW, Fischman MW, Pedroso JJ, et al. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav 1987;28:459—94.
14.Azilect (rasagiline mesylate) tablets. Kansas City, MO: Teva Neurosciences, Inc.; 2014 May.
15.Elavil® (amitriptyline) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2000 Dec.
16.Chan JC, Cockram CS, Critchley JA. Drug-induced disorders of glucose metabolism. Mechanisms and management. Drug Saf 1996;15:135—57.
17.Halothane, USP package insert. North Chicago, IL: Abbott Laboratories; 1998 Mar.
18.Tenuate® (diethylpropion hydrochloride) package insert. Bridgewater, NJ: Aventis Pharmaceuticals; 2003 Nov.
19.Strattera® (atomoxetine) package insert. Indianapolis, IN: Eli Lilly and Company; 2008 May.
20.Serevent® Diskus (salmeterol xinafoate inhalation powder) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2008 Mar.
21.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial.
22.O’Neill PM, Peterson, CA. Weight Loss and Depression in Overweight/Obese Subjects With a History of Depression Receiving Phentermine and Topiramate Extended-Release. Presented at the 166th Annual Meeting of the American Psychiatric Association (APA
23.Bostwick JM, Brown TM. A toxic reaction from combining fluoxetine and phentermine. J Clin Psychopharmacol 1996;16:189—90.
24.Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary disease. J Pharmacol Exp Ther. 2006;318:604-610.
25.Qsymia (phentermine and topiramate extended-release) package insert. Mountain View, CA: Vivus, Inc.; 2014 Sept.
26.Allison DB, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
27.Brintellix (vortioxetine tablets) package insert. Deerfield, IL: Takeda Pharmacueticals America, Inc.; 2014 Jul.
28.Suprenza (phentermine hydrochloride) package insert. Cranford, NJ: Akrimax Pharmaceuticals; 2011 Oct.
29.Phentermine hydrochloride package insert. Newtown, PA: KVK-Tech Inc; 2010 April.
30.Lomaira (phentermine hydrochloride) package insert. Newton, PA: KVK-Tech, Inc.; 2016 Sept.
31.Hendricks EJ, Srisurapanont M, Schmidt SL, et al. Addiction potential of phentermine prescribed during long-term treatment of obesity. Int J Obes (Lond). 2014;38:292-298.
Be the first to review “Phentermine HCl Capsules”
General Inquiries
There are no inquiries yet.









Reviews
There are no reviews yet.