- Description
- Reviews (0)
- Store Policies
- Inquiries
Description
Overview of Estradiol Cypionate Injection
-
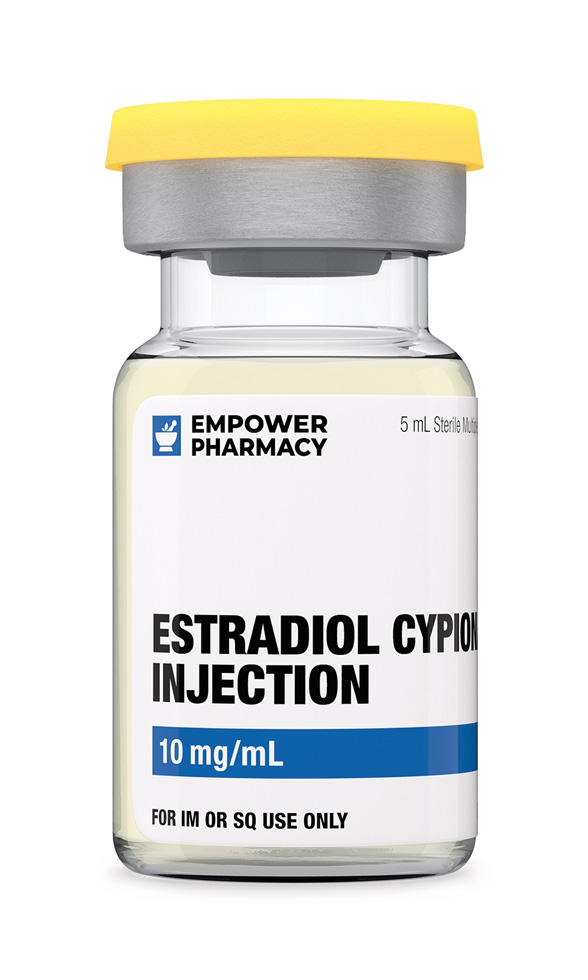
Dosage Strengths of Estradiol Cypionate Injection
-
Commercial: 5 mg/mL 5 mL Vial (Cottonseed Oil)
Compounded: 10 mg/mL 5 mL Vial (Grapeseed Oil)
Produced primarily in the ovaries, corpus luteum, placenta and, to a lesser extent, the liver and the heart, estrogen is an essential hormone in the gonadal development and function of women, especially those in the reproductive age group. Estrogen is also found in males, though at a significantly lesser concentration than in females. It has three primary physiologically active forms namely estrone (E1), 17 Beta estradiol (E2), and estriol (E3). Of these three active forms, estradiol is the most potent as well as predominant in women who are post -pubertal and premenopausal.
The effects of estradiol in the body are mediated by alpha and beta estrogen receptors. The distribution of these alpha and beta estrogen receptors on various organs in the body dictate the varied actions of estradiol. Some of the many roles of estradiol in the human body include:
- Development of breast tissue during puberty.
- Development of the mammary ducts during pregnancy.
- Stimulation of lactation in breastfeeding mothers.
- Fusion of epiphyseal growth plates at puberty as well as the inactivation of osteoclastic activity, thereby minimizing the risks of developing osteoporosis.
- Maintenance of epithelial cells in the vulva and vagina in women of reproductive age.
- Reduction in blood levels of low-density lipoproteins (LDL) and serum cholesterol as well as an increase in high-density lipoproteins (HDL) and triglyceride levels. This reduces the risk of developing coronary artery disease.
- A negative feedback effect resulting in the reduction in blood levels of follicular stimulating hormone (FSH) during at the onset of female menstruation.
Estradiol has several routes of administration depending on clinician and patient preferences, and the clinical indication. Common routes of estradiol administration are oral, topical (transdermal), intra-muscular (as depot preparations), and vaginal. Depending on its route of administration, estradiol has a variable hepatic first pass effect which plays a significant role in its bioavailability.
The enzyme Aromatase, a member of the Cytochrome P450 supergroup of enzymes, is responsible for the production of estradiol from its precursor, estrogen. It is located in several different sites on the body such as in the ovaries and testes, liver, skin, and endometrium, among others. Once produced, estradiol exerts their effects on the various organs and tissues within the body through the alpha and beta estrogen receptors. These estrogen receptors, part of a larger family of nuclear hormonal receptors, modulate the expression of specific genes by binding to particular DNA regulatory sequences. The gene modulation ability of estrogen receptors enables them to regulate various physiological functions within the human body. The receptor genes for the different estrogen receptors are located on different chromosomes; the alpha gene, known as ESR1, is located on chromosome 6 while the beta gene ESR2 is located on chromosome 14.
How estradiol performs its actions within the human body depends on which estrogen receptor type is stimulated. Estrogen receptor alpha is present mainly in the breasts, uterus, theca cells of the ovary, bone, male testes, prostate, liver, and adipose tissue; stimulation of this receptor type triggers responses in these organs. In contrast, estrogen receptor beta is found mainly in the bladder, granulosa cells of the ovary, colon, adipose tissue, and within the immune system.
As earlier stated, estradiol has multiple routes of administration namely oral, transdermal, intramuscular, and vaginal. In the bloodstream, estradiol binds to the plasma proteins albumin and Sex Hormone Binding Globulin (SHBG). It is generally distributed throughout the body, with higher concentrations in organs or tissues with the estrogen receptors alpha and beta.
In the liver, estradiol undergoes a first pass effect where it is broken down into estrone and estriol before it is excreted. This hepatic first pass effect occurs primarily when estradiol is orally administered. Estradiol administered through the transdermal or intramuscular route bypass the liver and, therefore, are not subject to the first pass effect. As a result of the hepatic first pass effect, the bioavailability of estradiol varies significantly depending on the route of administration. Orally administered estradiol has a lower bioavailability due to its extensive metabolism in the liver. This is in contrast to transdermal or intramuscular estradiol which has a higher bioavailability since it bypasses the liver.
In addition to hepatic metabolism, oral estradiol is also metabolized in the intestines to estrone and other estrone conjugates such as estrone sulfate, estrone glucuronide, and estradiol sulfate. This is a reversible process and some of the estradiol metabolites are converted back to estradiol. The estrone conjugates serve as a biological reservoir for estradiol; as such, orally administered estradiol typically has a significantly longer half life compared to estradiol administered through other routes. Following hepatic and intestinal metabolism, estradiol metabolites are excreted out of the body through urine and in stool.
There are certain circumstances under which estradiol should not be administered or, if administered, should be done with extreme caution. Some of the absolute or relative contraindications to estradiol administration include:
- Hypersensitivity: Estradiol should not be administered to individuals with demonstrated hypersensitivity to estradiol or any of its product ingredients
- Estrogen-dependent breast cancer: Individuals with breast cancer that are triggered by estrogen should not receive estradiol as this may lead to a worsening of the cancer.
- Ovarian cancer: Similar to breast cancer, the severity and spread of ovarian cancer may be worsened as a result of exogenous estradiol administration.
- Cerebrovascular disease: Estradiol administration can worsen symptoms in individuals with an active cerebrovascular disease such as a transient ischemic attack or a stroke. It is also contraindicated in individuals with a previous history of cerebrovascular disease.
- Coronary artery disease: Estradiol is contraindicated in individuals with a current or previous history of coronary artery disease such as myocardial infarction.
- Peripheral vascular disease: Vascular conditions such as thrombophlebitis, thromboembolism, deep vein thrombosis as well as pulmonary embolism are contraindications to estradiol administration.
- Hypercoagulable disease: Estradiol may worsen the signs and symptoms associated with hypercoagulable diseases such as Factor V Leiden syndrome, Protein C deficiency, Protein S deficiency, and metastatic diseases.
- Uterine disorders: Uterine conditions such as leiomyomas, commonly referred to as uterine fibroids, and endometriosis are relative contraindications to the administration of estradiol.
- Liver and gallbladder disease: Given the significance of the liver in the metabolism of estradiol, it is contraindicated in individuals with an active hepatic disease such as liver cirrhosis, hepatic adenoma, and hepatocellular carcinoma.
- Surgery: Due to the increased risk of thromboembolism, estradiol administration should be discontinued at least 4 – 6 weeks prior to any surgery that may require prolonged immobilization afterwards.
- Systemic Lupus Erythematosus (SLE): Individuals with SLE may have an increased likelihood of developing thromboembolic disorders and so estradiol should be used with caution.
- Hypocalcemia and hypoparathyroidism: Estrogen-induced hypocalcemia may occur in women suffering from hypoparathyroidism and, as such, caution should be exercised when administering estradiol. Estradiol administration may also worsen symptoms in individuals suffering from hypocalcemia.
- History of tobacco use: Estradiol should be administered with care to individuals with a history of tobacco use as this may increase the risk pf developing thromboembolic disorders.
Enough studies are not available to demonstrate the efficacy and safety of estradiol administration in neonate, infants, and children. Estradiol should generally be avoided pre-pubescent children because of the risk of premature epiphyseal closure.
Pregnancy is an absolute contraindication for the administration of exogenous estradiol as it freely crosses the placenta. Women who are pregnant should not receive estradiol, and in those who become pregnant while on estradiol therapy, it should be discontinued as soon as possible. Studies have demonstrated the increased likelihood of fetal abnormalities in women who received estradiol during pregnancy. Some of the fetal abnormalities that may arise from estradiol use in pregnancy are limb defects, cardiovascular defects, and urogenital abnormalities such as hydrocele and hypospadias. In women who were inadvertently on estrogen and progesterone contraceptives during early pregnancy, studies have not indicated an increase in the likelihood of fetal abnormalities.
Estradiol administration should be used with caution in breastfeeding mothers as detectable amounts have been found in breast milk. Estrogen administration during lactation has been shown to result in a decrease in the quantity as well as quality of breast milk; it should, therefore, be avoided as much as possible during pregnancy.
General side effects that may occur as a result of estradiol therapy are breast tenderness, nausea and vomiting, stomach cramps, skin hyperpigmentation, vaginal itching, and breakthrough uterine bleeding. In addition to these mild symptoms, individuals on estradiol may also experience more serious adverse effects; these include:
- Anaphylaxis: Individuals may develop anaphylactic reactions as a result of hypersensitive reactions to estradiol or its products.
- Cardiovascular disorders: There is a positive association between estradiol administration and the likelihood of developing cardiovascular disorders like venous thromboembolism, stroke, and myocardial infarction.
- Malignant carcinomas: There is an increased risk of developing endometrial, breast, or ovarian cancers in women on estradiol therapy.
- Visual abnormalities: Some patients receiving estradiol have reported instances of retinal vascular thrombosis.
- Hypercalcemia: Estradiol administration in individuals with breast cancer and bone metastases may result in severe hypercalcemia.
- Endometriosis exacerbation: Symptoms of endometriosis may be worsened by estradiol administration. In some instances, there has been a malignant transformation of endometrial tissue. In women with leiomyomas, estradiol may cause an increase in their sizes.
Store this medication at 68°F to 77°F (20°C to 25°C) and away from heat, moisture and light. Keep all medicine out of the reach of children. Throw away any unused medicine after the beyond use date. Do not flush unused medications or pour down a sink or drain.
Learn how to prepare medication for self-administered injection.
1.Cui, J., Shen, Y., Li, R., “Estrogen synthesis and signaling pathways during aging: from periphery to brain”, Trends in molecular medicine, vol.19 issue 3, pp 197–209, 2013. Available: https://doi.org/10.1016/j.molmed.2012.12.007.
2.Roby, K.F., “17 Beta Estradiol”, Reference Module in Biological Sciences. Available: https://www.sciencedirect.com/science/article/pii/B978012801238398019X
3.Reed BG, Carr BR, “The Normal Menstrual Cycle and the Control of Ovulation.” [Updated 2018 Aug 5].. Endotext [Internet]. 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279054/
4.Stevenson JC, “Type and route of estrogen administration”, Climacteric: The Journal of the International Menopause Society, vol. 12 issue Suppl 1, pp 86-90. Available: https://www.tandfonline.com/doi/abs/10.1080/13697130903007389?journalCode=icmt20
5.Lee HR, Kim TH, Choi KC, “Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse”, Lab Animal Research, vol.28 issue 2, June 2012. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3389841/
6.Paterni, I., Granchi, C.,Katzenellenbogen, J.A., “Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential”, Steroids, vol.0, November 2014. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4192010/#:~:text=The%20physiological%20functions%20of%20estrogenic,to%20associated%20DNA%20regulatory%20sequences.
7.Cui, J., Shen, Y., Li, R, “Estrogen synthesis and signaling pathways during aging: from periphery to brain.” Trends in molecular medicine, vol.19 issue 3, pp.197–209, 2013. Available: https://doi.org/10.1016/j.molmed.2012.12.007
8.Kuhl, H., “Pharmacology of estrogens and progestogens: influence of different routes of administration” Climacteric: The Journal of the International Menopause Society, vol. 3 issue Suppl 1, pp. 3-63
9.”Estradiol – Drug Summary”. Prescribers’ Digital Reference. Available: https://www.pdr.net/drug-summary/Estradiol-estradiol-1966.1610
11.Hemminki, E., Gissler M., Toukomaa, H., “Exposure to female hormone drugs during pregnancy: effect on malformations and cancer” British Journal of Cancer, vol.80 issue 7, pp. 1092-1097, 1999. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2363045/pdf/80-6690469a.pdf
10.”Estradiol”. Available: https://www.drugs.com/pro/estradiol.html#LINK_3f333729-d1ab-4413-883b-9f6c50383330
Be the first to review “Estradiol Cypionate Injection”
General Inquiries
There are no inquiries yet.








Reviews
There are no reviews yet.